Containment and glovebox technology, including in box processes.
The CAN team has many years of experience in the nuclear and nuclear medicines industry specialising in the area of containment.
Our clients engage us at the concept stage which normally includes an assessment of the internal process and selection of the most appropriate equipment and instrumentation. We employ the Aquila Influence diagram to substantiate our decision making. This process equipment often has to be modified or re-engineered for use within the containment suite.
We have the ability to undertake cold handling trials of processes including maintenance challenges within our full scale Multi Functional Cell (MFC) before proceeding into full detail manufacturing drawings.


- Feasibility studies and cost options
- Engineering substantiation and validation
- Concept design and detail schemes
- Full size mockup for handling/ergonomics trials and human factors assessment
- Budget pricing from concept designs and detail schemes
- Detail design and manufacturing drawings for gloveboxes and the complete process
- Full manufacturing specification including NDT
- Manufacture supply chain and procurement
- Validation of containment integrity using Aquila leak testing equipment
- Full assembly and factory acceptance tests (FATS)
- Installation and site acceptance tests
- Lifetime Quality Records
- Leak rates to 0.05%
- Full active validation design and selection of dampers, HEPA filters and control system
- Receipt and export of active material via DPTE, bagging or other bagless transfer
- Handling and movement of product inside containment – manually- remotely or semi remote
- Cutting open containers (normally waste)
- Measurement of product characteristics – mass – size- activity – contents
- Repackaging product streams
- Size reduction and compaction
- Welding and NDT
- Low and High temperature processes
- Chemical processes including synthesis and dispensing for medical isotopes
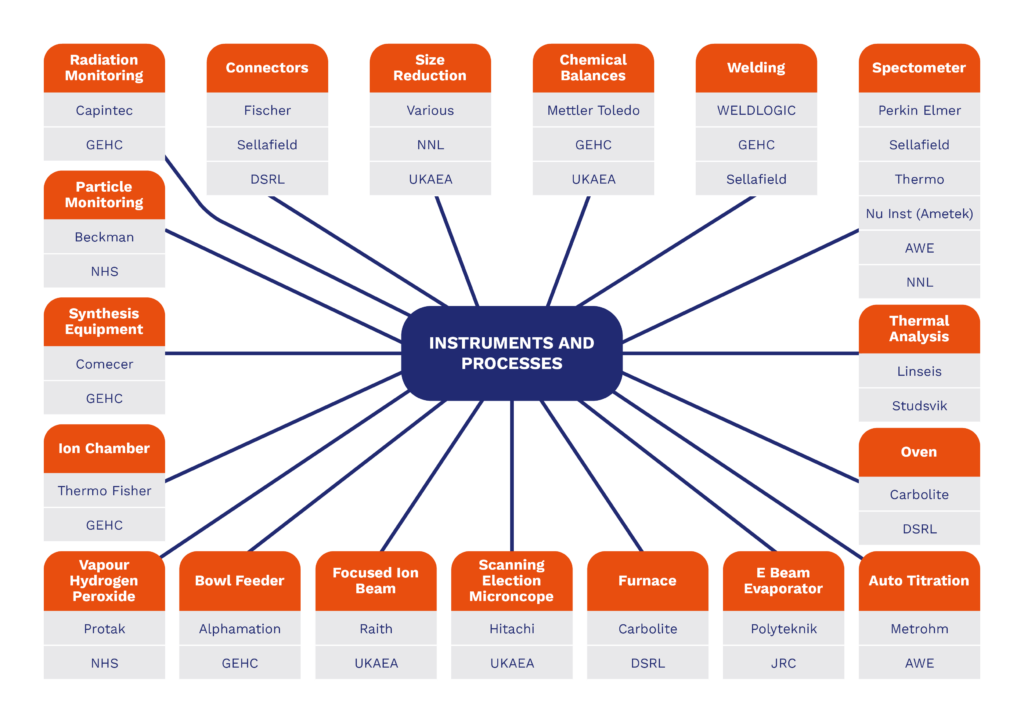
- Nuclearisation of instrumentation – examples are given below
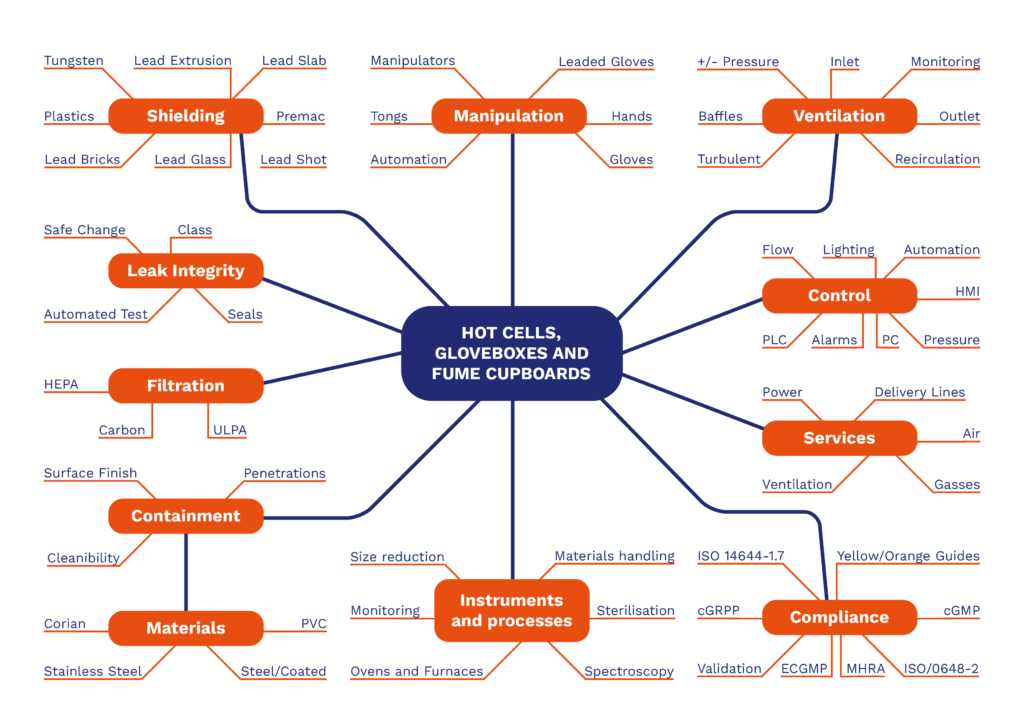
Influence Diagram
Instruments and Process Integration